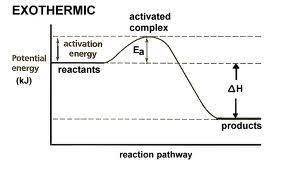
Which statement is true about the potential energy diagram for an exothermic reaction? the products have less potential energy than reactants. the energy value remains the same throughout the diagram. the graph starts at a lower energy value and ends at a higher energy value. the potential energy of the products is equal to the potential energy of the reactants

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, Luzperez09
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 21.06.2019 19:00, anonymous176
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Do you know the correct answer?
Which statement is true about the potential energy diagram for an exothermic reaction? the products...
Questions in other subjects:

Social Studies, 23.07.2020 21:01

Mathematics, 23.07.2020 21:01


Mathematics, 23.07.2020 21:01

English, 23.07.2020 21:01



Chemistry, 23.07.2020 21:01