
Chemistry, 21.11.2019 09:31, rubianny03
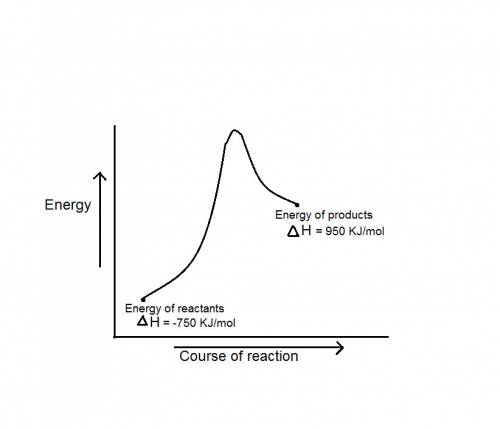
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statement is correct about the reaction?
it is endothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is endothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2


Chemistry, 22.06.2019 23:00, soccerplayer17
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
Do you know the correct answer?
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statemen...
Questions in other subjects:


English, 26.06.2019 23:00

Chemistry, 26.06.2019 23:00


Social Studies, 26.06.2019 23:00



English, 26.06.2019 23:00



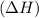 comes out to be positive.
comes out to be positive.



