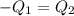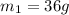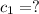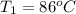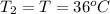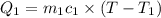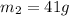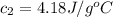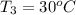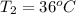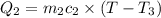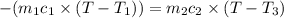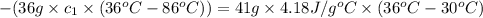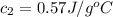When 36 g of a metal at 86 ◦c is added to
41 g of water at 30 ◦c, the temperature of the
...

Chemistry, 04.02.2020 11:52, paxbro3986
When 36 g of a metal at 86 ◦c is added to
41 g of water at 30 ◦c, the temperature of the
water rises to 36 ◦c. what is the specific heat
capacity of the metal? assume no heat was
lost to the surroundings.
answer in units of j/g ·◦c
.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 21.06.2019 22:00, braydentillery1221
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
Do you know the correct answer?
Questions in other subjects:



Geography, 10.10.2019 00:30






Chemistry, 10.10.2019 00:30