
Chemistry, 09.10.2019 18:00, auviannadority13
Aconcept map for four types of intermolecular forces and a certain type of bond is shown.
which of the following correctly identifies the intermolecular force represented by d and compares its strength relative to the intermolecular force represented by c?
a. d represents ion-dipole forces, which are weaker than the force represented by c.
b. d represents hydrogen bonding, which is weaker than the force represented by c.
c. d represents ion-dipole forces, which are stronger than the force represented by c.
d. d represents hydrogen bonding, which is stronger than the force represented by c.
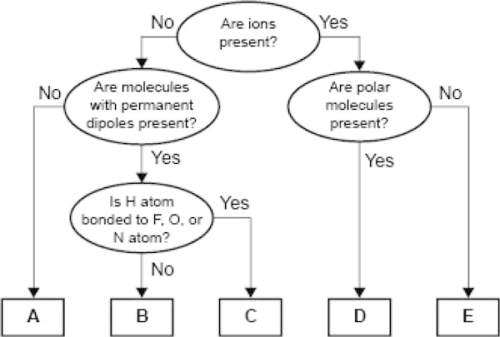

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
Do you know the correct answer?
Aconcept map for four types of intermolecular forces and a certain type of bond is shown.
Questions in other subjects:

English, 16.03.2020 20:15


Business, 16.03.2020 20:15

Chemistry, 16.03.2020 20:15


Mathematics, 16.03.2020 20:15

Mathematics, 16.03.2020 20:15



Health, 16.03.2020 20:15







