
Chemistry, 27.01.2020 20:31, issjzjjsmsm
The specific heat capacity (c) of a substance is the thermal energy required
to raise one gram of the substance by one degree celsius. the total amount
of thermal energy (q) added to a sample of a substance can be calculated
by multiplying the specific heat capacity (c) by the mass of the sample (m)
and the temperature change (t): q c m t. it takes 125 kilojoules (kj)
of energy to heat a cup of water, increasing its temperature by 1°c. what
is the temperature change in °c of the cup of water after 750 kj of energy
are used? s

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 04:00, miamassimino
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
Do you know the correct answer?
The specific heat capacity (c) of a substance is the thermal energy required
to raise one gram...
to raise one gram...
Questions in other subjects:

Health, 29.04.2021 07:50

Business, 29.04.2021 07:50




Mathematics, 29.04.2021 07:50

History, 29.04.2021 07:50



Mathematics, 29.04.2021 07:50


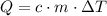 .
. nor
nor 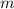 is given.
is given.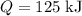 to this cup of water of mass
to this cup of water of mass 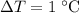 .
.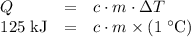
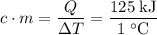 .
.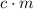 in the second calculation. Here's how:
in the second calculation. Here's how: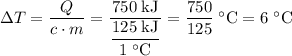 .
.



