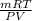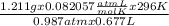Chemistry, 29.01.2020 03:59, svarner2001
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperature in the laboratory is 296 k, and the air pressure is 0.987 atm. calculate the molar mass of the gas.
a) 23.7 g/mol
b) 44.0 g/mol
c) 58.5 g/mol
d) 82.3 g/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 23.06.2019 05:00, lifeoflashay1659
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1

Chemistry, 23.06.2019 12:00, Pointjazzyqueen602
How can nonpolar molecule contain polar covalent bonds
Answers: 1

Chemistry, 23.06.2019 12:30, lindseylewis313
When utilizing a transmission electron microscope, why is it necessary to stain the specimen with heavy metal salts?
Answers: 1
Do you know the correct answer?
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperatu...
Questions in other subjects:


Biology, 23.05.2020 03:58

English, 23.05.2020 03:58


English, 23.05.2020 03:58


Mathematics, 23.05.2020 03:58

Business, 23.05.2020 03:58

Mathematics, 23.05.2020 03:58


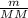 ) RT → MM =
) RT → MM =