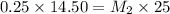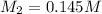Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 23.06.2019 04:31, saladdressing1425
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
Do you know the correct answer?
If 14.50 ml of 0.25 m sodium hydroxide reacts completely with 25.0 ml of hydrochloric acid, what is...
Questions in other subjects:

Mathematics, 11.12.2021 09:40

Mathematics, 11.12.2021 09:40







Mathematics, 11.12.2021 09:40


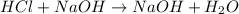
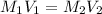
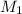 = Molarity of base= 0.25 M
= Molarity of base= 0.25 M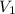 = volume of base= 14.50 ml
= volume of base= 14.50 ml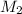 = Molarity of acid = ? M
= Molarity of acid = ? M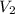 = volume of acid = 25 ml
= volume of acid = 25 ml