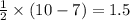Chemistry, 04.02.2020 02:58, IIHarmonyII
Use molecular orbital theory to complete this table by filling flanks, 0,1,2,3, or 4nf = (? 1s) *) ) *) (? 2p) 2p*) bonding order=nf+ =(? 1s) *) (? 2s*) 2p) ) 2p*) bonding order=nf- = (? (? 1s* (? 2s) * (? (? 2p) 2p*) bonding order=

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, angeljohnson2081
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1


Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1
Do you know the correct answer?
Use molecular orbital theory to complete this table by filling flanks, 0,1,2,3, or 4nf = (? 1s) *) )...
Questions in other subjects:




History, 18.08.2019 08:30



Mathematics, 18.08.2019 08:30

Mathematics, 18.08.2019 08:30


Mathematics, 18.08.2019 08:30


![(\sigma_{1s}),(\sigma_{1s}^*),(\sigma_{2s}),(\sigma_{2s}^*),(\sigma_{2p_z}),[(\pi_{2p_x})=(\pi_{2p_y})],[(\pi_{2p_x}^*)=(\pi_{2p_y}^*)],(\sigma_{2p_z}^*)](/tpl/images/0498/8593/33b96.png)
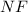 molecule = 7 + 9 = 16
molecule = 7 + 9 = 16![(\sigma_{1s})^2,(\sigma_{1s}^*)^2,(\sigma_{2s})^2,(\sigma_{2s}^*)^2,(\sigma_{2p_z})^2,[(\pi_{2p_x})^2=(\pi_{2p_y})^2],[(\pi_{2p_x}^*)^1=(\pi_{2p_y}^*)^1],(\sigma_{2p_z}^*)^0](/tpl/images/0498/8593/1c6dd.png)
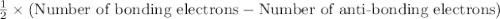
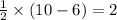
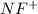 molecule = 7 + 9 - 1 = 15
molecule = 7 + 9 - 1 = 15![(\sigma_{1s})^2,(\sigma_{1s}^*)^2,(\sigma_{2s})^2,(\sigma_{2s}^*)^2,(\sigma_{2p_z})^2,[(\pi_{2p_x})^2=(\pi_{2p_y})^2],[(\pi_{2p_x}^*)^1=(\pi_{2p_y}^*)^0],(\sigma_{2p_z}^*)^0](/tpl/images/0498/8593/a69fc.png)
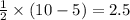
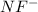 molecule = 7 + 9 + 1 = 17
molecule = 7 + 9 + 1 = 17![(\sigma_{1s})^2,(\sigma_{1s}^*)^2,(\sigma_{2s})^2,(\sigma_{2s}^*)^2,(\sigma_{2p_z})^2,[(\pi_{2p_x})^2=(\pi_{2p_y})^2],[(\pi_{2p_x}^*)^2=(\pi_{2p_y}^*)^1],(\sigma_{2p_z}^*)^0](/tpl/images/0498/8593/6101d.png)