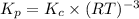Consider the following equilibrium:
4fe(s) + 3 o2(g) < -- --> 2fe2o3(s);
<...

Chemistry, 04.02.2020 04:43, kris22elizondop9v1bb
Consider the following equilibrium:
4fe(s) + 3 o2(g) < -- --> 2fe2o3(s);
which of the following equations is wrong?
kp=kc(rt)-5
kc=[o2]-3
kp= kc(rt)-3
kp = po2-3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
Do you know the correct answer?
Questions in other subjects:






Biology, 24.08.2019 01:30


Mathematics, 24.08.2019 01:30

Computers and Technology, 24.08.2019 01:30

History, 24.08.2019 01:30


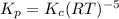
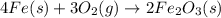
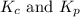 is given by:
is given by:![K_c=\frac{1}{[O_2]^3}](/tpl/images/0499/0672/4f5e0.png)
![K_p=\frac{1}{[O_2]^3}](/tpl/images/0499/0672/d3f6c.png)
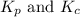 is given by the expression:
is given by the expression: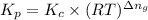
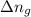 = number of moles of gaseous products - number of moles of gaseous reactants
= number of moles of gaseous products - number of moles of gaseous reactants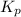 is:
is: