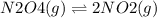Chemistry, 02.02.2020 10:44, kimlyn58p0wyn0
Which situation would cause the following equilibrium reaction to shift to the right? (1 point) n2o4 (g)two arrows stacked on top of each other. the top arrow points to the right. the bottom arrow points to the left. 2 no2
increase the pressure
add n2o4
add no2
decrease the volume

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:20, zymikaa00
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
Do you know the correct answer?
Which situation would cause the following equilibrium reaction to shift to the right? (1 point) n2o...
Questions in other subjects: