
Chemistry, 21.01.2020 15:31, AnxiousKid
Janet observes that the shape of a dented table tennis ball is restored by heating the ball gently in warm water. which of the following gas laws is used to understand the observation made by janet and why?
the high temperature lowers volume according to boyle's law because this law describes how a gas will behave when the number of moles remains constant.
the high temperature raises volume according to boyle's law because this law describes how a gas will behave when the number of moles remains constant.
the high temperature lowers the volume of air inside the ball according to charles's law because this law describes how a gas will behave at constant pressure.
the high temperature raises the volume of air inside the ball according to charles's law because this law describes how a gas will behave at constant pressure.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2



Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
Do you know the correct answer?
Janet observes that the shape of a dented table tennis ball is restored by heating the ball gently i...
Questions in other subjects:


Chemistry, 03.08.2021 15:50


English, 03.08.2021 15:50

Social Studies, 03.08.2021 15:50


Physics, 03.08.2021 15:50


Advanced Placement (AP), 03.08.2021 15:50


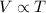 .
.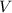 is the volume of an ideal gas, and
is the volume of an ideal gas, and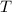 is its temperature in degrees Kelvins.
is its temperature in degrees Kelvins.




