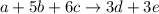Chemistry, 04.11.2019 21:31, dalejacksoniip5yf4y
The balanced equation for a hypothetical reaction is a + 5b + 6c → 3d + 3e. what is the rate law for this reaction? rate = k [a][b]5[c]6 rate = k [a][b][c] rate = k [d]3[e]3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 21.06.2019 20:30, notkeandre9
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1


Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1
Do you know the correct answer?
The balanced equation for a hypothetical reaction is a + 5b + 6c → 3d + 3e. what is the rate law for...
Questions in other subjects:










English, 02.03.2020 22:56


![Rate=k[a][b]^5[c]^6](/tpl/images/0359/2871/fe35d.png)