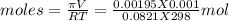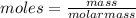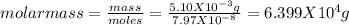Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 06:10, tammydbrooks43
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
Do you know the correct answer?
Asolution was made by dissolving 5.10 mg of hemoglobin in water to give a final volume of 1.00 ml. t...
Questions in other subjects:

Mathematics, 18.01.2021 22:50

Mathematics, 18.01.2021 22:50

Mathematics, 18.01.2021 22:50

Business, 18.01.2021 22:50

English, 18.01.2021 22:50




Mathematics, 18.01.2021 22:50

History, 18.01.2021 22:50