
Chemistry, 25.09.2019 01:00, genyjoannerubiera
In part iii, the phenolphthalein indicator is used to monitor the equilibrium shifts of the ammonia/ammonium ion system. the phenolphthalein equilibrium established with water is hph(aq)(colorless) + h2o (l) h3o+ (aq) + ph-(aq)(pink or red). you compared the color of the solutions in three test tubes that initially contained 3 ml of 0.1 m ammonium hydroxide and a few drops of phenolphthalein indicator. in the first test tube, you added 1 m nh4cl dropwise. what color change was observed and what did this color change indicate about the shift in the phenolphthalein equilibrium? a. the solution turned a more intense pink or red color indicating that the phenolphthalein equilibrium shifted to the left, producing more of the pink or red colored hph.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1


Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1
Do you know the correct answer?
In part iii, the phenolphthalein indicator is used to monitor the equilibrium shifts of the ammonia/...
Questions in other subjects:

Geography, 22.11.2019 15:31


History, 22.11.2019 15:31

History, 22.11.2019 15:31


Business, 22.11.2019 15:31

Mathematics, 22.11.2019 15:31




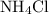 is a salt soluble in water.
is a salt soluble in water. 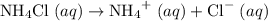 .
. .
. 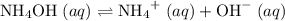 .
.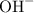 and
and 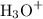 ions.
ions.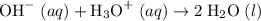 .
.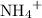 ions in the solution. Some of the
ions in the solution. Some of the 



