
Chemistry, 05.02.2020 04:54, rileyallen4186pd5tgy
Consider the balanced chemical reaction below. when the reaction was carried out, the calculated theoretical yield for carbon dioxide was 93.7 grams, but the measured yield was 88.3 grams. what is the percent yield?
fe2o3 + 3co --> 2fe + 3co2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3


Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
Do you know the correct answer?
Consider the balanced chemical reaction below. when the reaction was carried out, the calculated the...
Questions in other subjects:


Mathematics, 19.09.2019 15:00


Social Studies, 19.09.2019 15:00

Chemistry, 19.09.2019 15:00


English, 19.09.2019 15:00

Mathematics, 19.09.2019 15:00


Physics, 19.09.2019 15:00


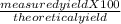
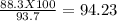 %
%



