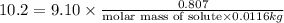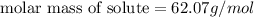Chemistry, 04.02.2020 20:46, Envious1552
Tert-butyl alcohol is a solvent with a kf of 9.10 ∘c/m and a freezing point of 25.5 ∘c. when 0.807 g of an unknown colorless liquid was dissolved in 11.6 g of tert-butyl alcohol, the solution froze at 15.3 ∘c. which of the following is most likely the identity of this unknown liquid? tert-butyl alcohol is a solvent with a of 9.10 and a freezing point of 25.5 . when 0.807 of an unknown colorless liquid was dissolved in 11.6 of tert-butyl alcohol, the solution froze at 15.3 .which of the following is most likely the identity of this unknown liquid? ethylene glycol (molar mass = 62.07 g/mol)1-octanol (molar mass = 130.22 g/mol)glycerol (molar mass = 92.09 g/mol)2-pentanone (molar mass = 86.13 g/mol)1-butanol (molar mass = 74.12 g/mol)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2


Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
Do you know the correct answer?
Tert-butyl alcohol is a solvent with a kf of 9.10 ∘c/m and a freezing point of 25.5 ∘c. when 0.807 g...
Questions in other subjects:

Mathematics, 08.03.2021 17:50

History, 08.03.2021 17:50





English, 08.03.2021 17:50

Mathematics, 08.03.2021 17:50





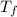 = change in freezing point
= change in freezing point = freezing point constant=
= freezing point constant=