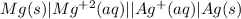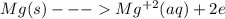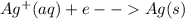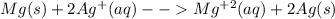An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) || aq^+(aq) | aq(s)
write a balanced redox equation for the cell using the oxidation and reduction half-reactions. (be sure to equalize charge by multiplying by the correct numbers before adding and simplifying)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, monsurviky
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 22.06.2019 08:40, alexisbcatlett14
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 23:00, genyjoannerubiera
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
Do you know the correct answer?
An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) ||...
mg(s) | mg^2+ (aq) ||...
Questions in other subjects:

Mathematics, 01.12.2020 04:00


English, 01.12.2020 04:00

History, 01.12.2020 04:00

Mathematics, 01.12.2020 04:00





Mathematics, 01.12.2020 04:00