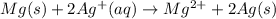An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) ||...

An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) || aq^+(aq) | aq(s)
write a blanked redox equation for the cell using the oxidation and reduction half reactions. (be sure to equalize charge by multiplying by the correct numbers before adding and simplifying)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1


Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 25.03.2021 21:50

Chemistry, 25.03.2021 21:50





History, 25.03.2021 21:50



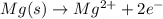 ....(1)
....(1)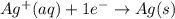 ...(2)
...(2)