
Chemistry, 07.10.2019 22:30, grantjaylynn
At very high temperatures, copper(ii) sulfate undergoes the reaction cuso4(s) --> cuo (s) + so3 (g)
what kind of reaction is this?
if 319.22 g cuso4(s) reacted completely in the reaction, how many grams of cuo(s) would be produced?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2
Do you know the correct answer?
At very high temperatures, copper(ii) sulfate undergoes the reaction cuso4(s) --> cuo (s) + so3...
Questions in other subjects:




English, 12.12.2019 05:31

Health, 12.12.2019 05:31




Advanced Placement (AP), 12.12.2019 05:31


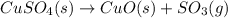
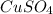 gives 1 mole of
gives 1 mole of 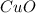 .
. 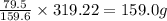 of
of 



