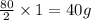When magnesium burns in air, it combines with oxygen to form magnesium oxide according to the following equation. what mass in grams of magnesium oxide is produced from 2.00 mol of magnesium?
2mg(s) + o2(g) -> 2mgo(s)
what is the given quantity?
what is the unknown quantity?
write the two conversion factors needed to solve the problem.
calculate the molar mass of the unknown using the given quantity and the two conversion factors.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, smhrosepetals
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 04:00, anonymous1813
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
Do you know the correct answer?
When magnesium burns in air, it combines with oxygen to form magnesium oxide according to the follow...
Questions in other subjects:

Mathematics, 07.02.2021 01:50

Mathematics, 07.02.2021 01:50


Chemistry, 07.02.2021 01:50

Biology, 07.02.2021 01:50


Mathematics, 07.02.2021 01:50

Chemistry, 07.02.2021 01:50

Mathematics, 07.02.2021 01:50


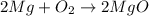
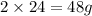 of magnesium produce=
of magnesium produce=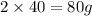 of MgO.
of MgO.