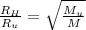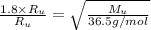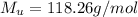Chemistry, 25.12.2019 03:31, majormkh10
describe graham's law. hydrogen chloride gas (hcl) diffuses 1.8 times faster than an unknown gas. determine the molar mass of the unknown gas. show your work or explain your answer, giving specific values used to determine the answer.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 07:30, fernandancon1872
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

Chemistry, 23.06.2019 08:10, andrewrangel63
An experiment is conducted to see if cats preferred skim milk or 2% milk. a cup of skim milkwas put out for 5 kittens and then measured how much the kittens drank over the course of aday. following a cup of 2% milk was purout for the skittens and then masured how much thekittens drank over the course of a day. the same kittens were used and the milk was served atthe same temperature. it was discovered that the cats liked the 2% milk more than the skimmilk. what is the dependent variable in this experiment?
Answers: 1
Do you know the correct answer?
describe graham's law. hydrogen chloride gas (hcl) diffuses 1.8 times faster than an unknown gas. de...
Questions in other subjects:


Mathematics, 08.07.2019 05:00


Mathematics, 08.07.2019 05:00

Mathematics, 08.07.2019 05:00

Computers and Technology, 08.07.2019 05:00

Health, 08.07.2019 05:00


Mathematics, 08.07.2019 05:00


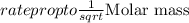
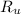 with molar mass of
with molar mass of 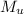
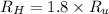 (given)
(given)