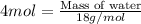Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
Do you know the correct answer?
C3h8 + 5o2 = 3co2 + 4h2o when 44.0 grams of propane (c3h8) under goes complete combustion, how many...
Questions in other subjects:






History, 14.12.2019 21:31



Mathematics, 14.12.2019 21:31

Mathematics, 14.12.2019 21:31


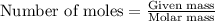 ....(1)
....(1)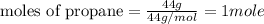
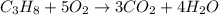
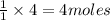 of water.
of water.