
Hello, everyone!
here is the problem i am stuck with.:
based on the “national ambient air quality objectives”, the acceptable hourly average concentration of carbon monoxide (co) is 35 mg/m^3.
find the acceptable concentration of co in ppm if the temperature is -30 °c and pressure is 0.92 atm. express the concentration as a percent by volume.
could someone me understand how to do it?
any input would be greatly appreciated!

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
Do you know the correct answer?
Hello, everyone!
here is the problem i am stuck with.:
based on the “national am...
here is the problem i am stuck with.:
based on the “national am...
Questions in other subjects:



Mathematics, 22.08.2019 16:30

English, 22.08.2019 16:30


History, 22.08.2019 16:30

Business, 22.08.2019 16:30

Mathematics, 22.08.2019 16:30



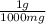 x
x 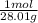
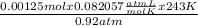
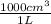 = 27.09 cm³
= 27.09 cm³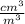 x
x 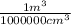 x 100%
x 100%



