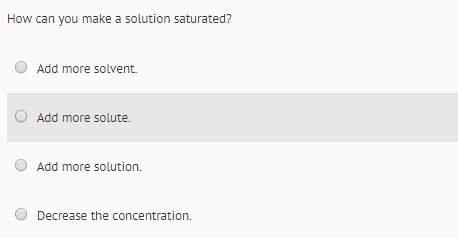
Chemistry, 15.10.2019 06:30, irenecupcake4348
Consider this second-order reaction: (a b c and rate = k[a]2). what will happen to the reaction rate if the concentration of a is doubled?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3


Chemistry, 23.06.2019 15:20, brandon0227
Which element below could be an isotope of berylliumsodium-10beryllium-10boron -9carbon-9
Answers: 2
Do you know the correct answer?
Consider this second-order reaction: (a b c and rate = k[a]2). what will happen to the reaction rat...
Questions in other subjects:


English, 21.05.2021 19:50



Mathematics, 21.05.2021 19:50

Mathematics, 21.05.2021 19:50


Chemistry, 21.05.2021 19:50