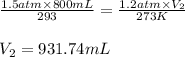Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

Chemistry, 22.06.2019 16:50, mathiscool7
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
Do you know the correct answer?
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation shoul...
Questions in other subjects:


SAT, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01


Biology, 20.09.2020 06:01

History, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01




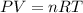
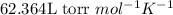
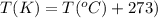
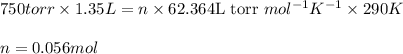
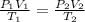
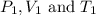 are initial pressure, volume and temperature of the gas
are initial pressure, volume and temperature of the gas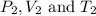 are final pressure, volume and temperature of the gas
are final pressure, volume and temperature of the gas