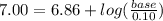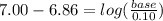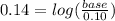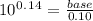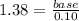Chemistry, 24.06.2019 10:00, mimireds8573
Suppose you wanted to make a buffer of exactly ph 7.00 using kh2po4 and na2hpo4. if the final solution was 0.10 m in kh2po4, what concentration of na2hpo4 would you need? (pka for h3po4, h2po−4, and hpo2−4 are 2.14, 6.86, and 12.40, respectively.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1


Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Do you know the correct answer?
Suppose you wanted to make a buffer of exactly ph 7.00 using kh2po4 and na2hpo4. if the final soluti...
Questions in other subjects:












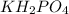 acts as a weak acid and
acts as a weak acid and 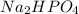 as a base which is pretty conjugate base of the weak acid we have.
as a base which is pretty conjugate base of the weak acid we have.