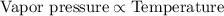Chemistry, 24.06.2019 15:00, drunkelmo04
There are four equal-sized bowls with equal amounts of milk. bowl a is covered, and the milk is at room temperature. bowl b is uncovered, and the milk is warmer than room temperature. bowl c is uncovered, and the milk is cooler than room temperature. bowl d is uncovered, and the milk is at room temperature. which bowl has the highest vapor pressure?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Do you know the correct answer?
There are four equal-sized bowls with equal amounts of milk. bowl a is covered, and the milk is at...
Questions in other subjects:



Computers and Technology, 11.01.2022 04:00





Mathematics, 11.01.2022 04:00