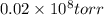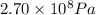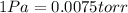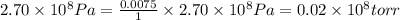Chemistry, 24.06.2019 20:30, hannahbrown889
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa ) to about 60,000 kpa (60,000,000 pa ). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 2.70×108 pa , what is its running pressure in torr? express the pressure numerically in torr.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, Tyrant4life
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
Do you know the correct answer?
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify...
Questions in other subjects:

French, 18.02.2021 22:30

Physics, 18.02.2021 22:30



Mathematics, 18.02.2021 22:30

Mathematics, 18.02.2021 22:30



Social Studies, 18.02.2021 22:30