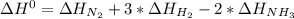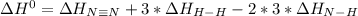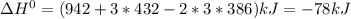Chemistry, 24.06.2019 21:30, gwoodbyrne
Given: n2 + 3h2 → 2nh3 bond bond energy (kj/mol) n≡n 942 h–h 432 n–h 386 use the bond energies to calculate the change in enthalpy for the reaction. the enthalpy change for the reaction is kilojoules.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2



Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
Do you know the correct answer?
Given: n2 + 3h2 → 2nh3 bond bond energy (kj/mol) n≡n 942 h–h 432 n–h 386 use the bond energies to c...
Questions in other subjects:


Mathematics, 09.04.2021 06:10

Mathematics, 09.04.2021 06:10

Mathematics, 09.04.2021 06:10



Biology, 09.04.2021 06:10

Mathematics, 09.04.2021 06:10



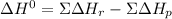 (2)
(2)