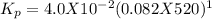Chemistry, 25.06.2019 02:00, taysomoneyyy
If kc = 4.0×10−2 for pcl3(g)+cl2(g)⇌pcl5(g) at 520 k , what is the value of kp for this reaction at this temperature?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
Do you know the correct answer?
If kc = 4.0×10−2 for pcl3(g)+cl2(g)⇌pcl5(g) at 520 k , what is the value of kp for this reaction at...
Questions in other subjects:

English, 03.07.2019 04:00


Mathematics, 03.07.2019 04:00



English, 03.07.2019 04:00


History, 03.07.2019 04:00

Physics, 03.07.2019 04:00


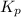 of the reaction at 520 K temperature.
of the reaction at 520 K temperature.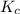 is
is 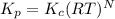 , where
, where