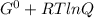Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, ReveenatheRaven2296
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3
Do you know the correct answer?
What is δg for the formation of solid uranium hexafluoride from uranium and fluorine at 25∘c when th...
Questions in other subjects:

Chemistry, 24.09.2020 20:01


Mathematics, 24.09.2020 20:01







Biology, 24.09.2020 20:01


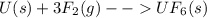
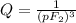
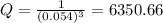
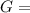 Δ
Δ