
Chemistry, 25.06.2019 05:30, sanociahnoel
If 3.8 moles of zinc metal react with 6.5 moles of silver nitrate, how many moles of silver metal can be formed, and how many moles of the excess reactant will be left over when the reaction is complete? unbalanced equation: zn + agno3 → zn(no3)2 + ag be sure to show all of your work.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, alexisfaithsmith
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2


Chemistry, 23.06.2019 08:30, aaliyahnv07
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
Do you know the correct answer?
If 3.8 moles of zinc metal react with 6.5 moles of silver nitrate, how many moles of silver metal ca...
Questions in other subjects:

Biology, 11.01.2020 20:31

Mathematics, 11.01.2020 20:31

Mathematics, 11.01.2020 20:31


Mathematics, 11.01.2020 20:31

Mathematics, 11.01.2020 20:31

Mathematics, 11.01.2020 20:31




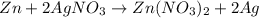
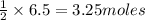 of zinc metal
of zinc metal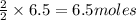 of silver metal.
of silver metal.




