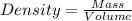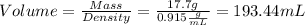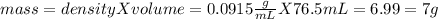Chemistry, 25.06.2019 05:30, phebusadrian01
Aliquid sample, with a density of .0915 g/ml has a mass of 17.7 grams. 1.) what is the volume of this sample (in ml)? 2.)what mass (in grams) of this sample would have a volume of 76.5 ml?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
Do you know the correct answer?
Aliquid sample, with a density of .0915 g/ml has a mass of 17.7 grams. 1.) what is the volume of th...
Questions in other subjects:

Chemistry, 18.11.2020 19:00



English, 18.11.2020 19:00

Mathematics, 18.11.2020 19:00


Computers and Technology, 18.11.2020 19:00