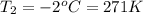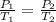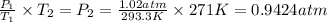Chemistry, 25.06.2019 11:00, MyChannelBruh6896
Asample of atmospheric gas collected at an industrial site is stored in a 250 ml amber glass bottle that has a pressure of 1.02 atm and a temperature of 20.3°c. it was placed in an ice chest whose internal temperature is -2.0°c. what will be the new pressure of the gas sample once the gas temperature in the jar equilibrates to that of the ice chest?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3


Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
Do you know the correct answer?
Asample of atmospheric gas collected at an industrial site is stored in a 250 ml amber glass bottle...
Questions in other subjects:


Chemistry, 21.10.2020 19:01





Mathematics, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01



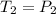
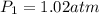 ,
, 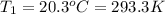
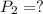 ,
,