
Chemistry, 25.06.2019 13:30, cloeybrown
Which of the following has the greatest electronegativity difference between the bonded atoms? a. a strong acid made of hydrogen and a halogen, such as hcl b. carbon bonded to a group 6 a (16) nonmetal chalcogen, such as in co c. a diatomic gas, such as nitrogen (n2).d. a group 1 alkali metal bonded to iodide, such as nai.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1


Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
Do you know the correct answer?
Which of the following has the greatest electronegativity difference between the bonded atoms? a. a...
Questions in other subjects:

Mathematics, 01.02.2021 05:30

Engineering, 01.02.2021 05:30

Spanish, 01.02.2021 05:30






History, 01.02.2021 05:30


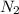 = Electronegativity value of nitrogen - electronegativity value of nitrogen
= Electronegativity value of nitrogen - electronegativity value of nitrogen



