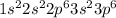Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
Do you know the correct answer?
The electron configuration of an element is shown below. 1s22s22p63s23p6 name the group this element...
Questions in other subjects:

Mathematics, 11.12.2019 07:31



Mathematics, 11.12.2019 07:31

Chemistry, 11.12.2019 07:31