
An ion of an isotope has a 2+ charge, an atomic mass of 56.9397 amu, 2 electrons at the n=4 energy level and 13 electrons at the n=3 energy level. determine a. atomic number b. mass number c. total number of electrons d. total number of s electrons e. total number of p electrons f. total number of d electrons

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 22.06.2019 19:30, jessixa897192
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
Do you know the correct answer?
An ion of an isotope has a 2+ charge, an atomic mass of 56.9397 amu, 2 electrons at the n=4 energy l...
Questions in other subjects:


English, 30.07.2019 20:00

Health, 30.07.2019 20:00


Business, 30.07.2019 20:00




Mathematics, 30.07.2019 20:00


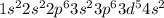
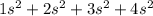 = 8
= 8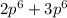 = 12
= 12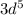 = 5
= 5




