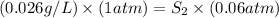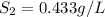1. the solubility of agno3 at 20°c is 222.0g agno3/100g h2o. what mass of agno3 can be dissolved in 250 g of water at 20°c? recall that solubility = mass of solute/ mass of solvent. 2. the solubility of methane, the major component of natural gas, in water at 20°c and 1.00 atm pressure is 0.026 g/l. if the temperature remains constant, what will be the solubility of this gas at 0.06 atm pressure? recall the relationship between solubility and pressure for gases: s1/p1 = s2/p2

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
Do you know the correct answer?
1. the solubility of agno3 at 20°c is 222.0g agno3/100g h2o. what mass of agno3 can be dissolved in...
Questions in other subjects:

English, 21.01.2021 19:20

Arts, 21.01.2021 19:20



Mathematics, 21.01.2021 19:20





Mathematics, 21.01.2021 19:20


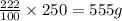
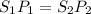 (at constant temperature)
(at constant temperature)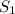 = initial solubility of methane gas = 0.026 g/L
= initial solubility of methane gas = 0.026 g/L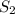 = final solubility of methane gas
= final solubility of methane gas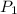 = initial pressure of methane gas = 1 atm
= initial pressure of methane gas = 1 atm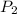 = final pressure of methane gas = 0.06 atm
= final pressure of methane gas = 0.06 atm