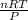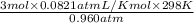1. what is the mass of 22.4 l of h2 at stp? a.) 1.01 grams b.) 2.02 grams c.) 11.2 grams d.) 22.4 grams 2. read the chemical equation. mg + hcl → mgcl2 + h2 how many liters of hydrogen gas is produced at 298 k and 0.960 atm if 3.00 moles of hydrochloric acid react with an excess of magnesium metal? a.) 38.2 liters b.) 42.6 liters c.) 66.7 liters d.) 76.4 liters

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, alexisfaithsmith
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2
Do you know the correct answer?
1. what is the mass of 22.4 l of h2 at stp? a.) 1.01 grams b.) 2.02 grams c.) 11.2 grams d.) 22.4 g...
Questions in other subjects:

Mathematics, 17.01.2022 14:00

Mathematics, 17.01.2022 14:00

Mathematics, 17.01.2022 14:00


Mathematics, 17.01.2022 14:00

Mathematics, 17.01.2022 14:00


Mathematics, 17.01.2022 14:00

Geography, 17.01.2022 14:00


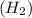 is 2.02 g/mol.
is 2.02 g/mol. 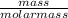
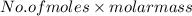
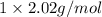
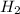 at STP is 2.02 grams.
at STP is 2.02 grams.