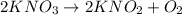Chemistry, 26.06.2019 17:00, jacobs5919
Achemical equation is shown below. kno3 → kno2 + o2 what are the coefficients that should be added to balance this equation? use complete sentences to explain your answer. explain how this chemical reaction demonstrates the conservation of mass.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
Do you know the correct answer?
Achemical equation is shown below. kno3 → kno2 + o2 what are the coefficients that should be added t...
Questions in other subjects:



Mathematics, 18.05.2021 21:40



Mathematics, 18.05.2021 21:40

English, 18.05.2021 21:40



Biology, 18.05.2021 21:40


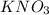 and
and  in order to balance a chemical reaction.
in order to balance a chemical reaction.