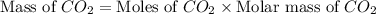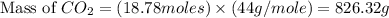Iron metal is obtained from the reaction of hematite [iron (iii) oxide, fe2o3] with carbon monoxide in a blast furnace. fe2o3 (s) + 3 co (g) > 2 fe (s) + 3 co2 (g) (a) calculate the number of grams of iron metal that can be obtained from 1.00 kg of hematite (assuming that you have enough co available for any reaction). feb) calculate the amount of co2 in grams that you you will get in this reaction, using the amount of hematite in (a). g co2

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
Do you know the correct answer?
Iron metal is obtained from the reaction of hematite [iron (iii) oxide, fe2o3] with carbon monoxide...
Questions in other subjects:

Chemistry, 02.12.2019 03:31


Mathematics, 02.12.2019 03:31


Physics, 02.12.2019 03:31

Mathematics, 02.12.2019 03:31

Mathematics, 02.12.2019 03:31

Mathematics, 02.12.2019 03:31



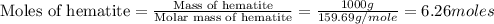
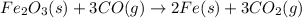
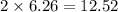 moles of iron
moles of iron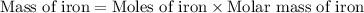
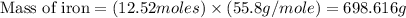
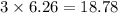 moles of carbon dioxide
moles of carbon dioxide