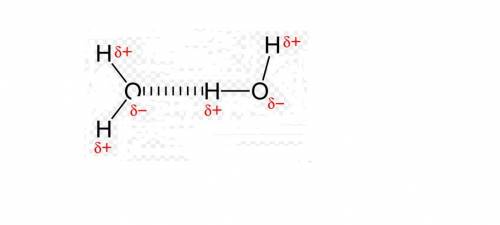
Which sentence best explains the high melting point, boiling point, and surface tension of water? a. the hydrogen atom on each water molecule is strongly attracted to the hydrogen atoms on nearby molecules. b. ions dissolved in the water cause nearby molecules to become temporarily polar. c. the water molecules are connected to each other with covalent bonds. d. water molecules that have lost electrons attract the water molecules that have gained electrons. e. the negative side of each water molecule is attracted to the positive sides of nearby molecules.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1
Do you know the correct answer?
Which sentence best explains the high melting point, boiling point, and surface tension of water? a...
Questions in other subjects: