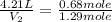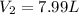Chemistry, 27.06.2019 03:00, paulesparsa6
If 27.1 g of ar(g) occupies a volume of 4.21 l, what volume will 1.29 moles of ne(g) occupy at the same temperature and pressure?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
Do you know the correct answer?
If 27.1 g of ar(g) occupies a volume of 4.21 l, what volume will 1.29 moles of ne(g) occupy at the s...
Questions in other subjects:

Mathematics, 30.12.2019 11:31

Biology, 30.12.2019 11:31



Mathematics, 30.12.2019 11:31

English, 30.12.2019 11:31



Social Studies, 30.12.2019 11:31

Biology, 30.12.2019 11:31


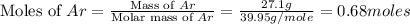
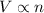
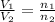
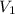 = volume of argon gas
= volume of argon gas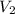 = volume of neon gas
= volume of neon gas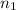 = number of moles of argon gas
= number of moles of argon gas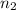 = number of moles of neon gas
= number of moles of neon gas