
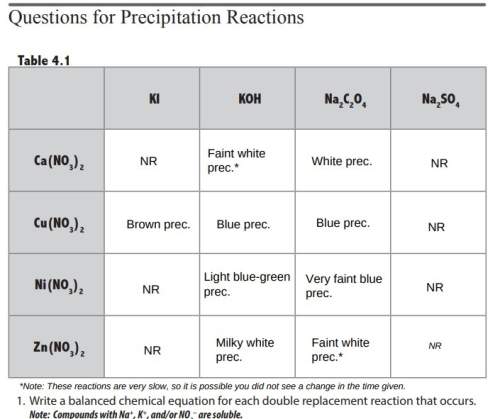
:d 1. write a balanced chemical equation for each double replacement reaction that occurs. note: compounds with na+, k+, and/or no3− are soluble. 2. why was there no reaction in some of the wells? (see introduction.) 3. how could you tell a ca(no3)2 solution from a zn(no3)2 solution? 4. how could you tell a cu(no3)2 solution from a ni(no3)2 solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, kevinhill185
Pauling and lewis questioned the extreme definitions of bonds. they wondered if bonds might be described somewhere in between the two extremes (covalent and ionic). on the basis of experimental data, pauling confirmed that bonds could be ionic, covalent, and for those, in between, exhibit a degree of ionic character. he theorized that the major factor was how strongly the atoms in the bond attracted the electrons. pauling called this factor - the tendency of an atom to attract electrons in a bond.
Answers: 2

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Do you know the correct answer?
:d 1. write a balanced chemical equation for each double replacement reaction that occurs. note: co...
Questions in other subjects:


Advanced Placement (AP), 24.06.2019 06:00

Mathematics, 24.06.2019 06:00




Biology, 24.06.2019 06:00

History, 24.06.2019 06:00


Mathematics, 24.06.2019 06:00






