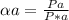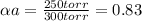Chemistry, 27.06.2019 14:30, JewelzSkullz
Substances a and b are both volatile liquids with p*a = 300 torr, p*b = 250 torr, and kb = 200 torr (concentration expressed in mole fraction). when xa = 0.9, bb = 2.22 mol kg−1, pa = 250 torr, and pb = 25 torr. calculate the activities and activity coefficients of a and b. use the mole fraction, raoult's law basis system for a and the henry's law basis system (both mole fractions and molalities) for b.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, themajesty9898
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
Do you know the correct answer?
Substances a and b are both volatile liquids with p*a = 300 torr, p*b = 250 torr, and kb = 200 torr...
Questions in other subjects:

Health, 03.11.2020 01:50

Mathematics, 03.11.2020 01:50

History, 03.11.2020 01:50

Mathematics, 03.11.2020 01:50


Chemistry, 03.11.2020 01:50


Computers and Technology, 03.11.2020 01:50

Biology, 03.11.2020 01:50

Mathematics, 03.11.2020 01:50