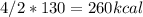Chemistry, 27.06.2019 15:30, hahalol123goaway
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced equation: 2cl2(g) + 7o2(g) + 130kcal -> 2cl2o7(g) a. 1040 kcal b. -260 kcal c. 260 kcal d. -1040 kcal ** if you could explain it as well, that would be much appreciated if not, thats okay too its multiple choice

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, rubimachuca1020
Given 7.65 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield?
Answers: 3


Chemistry, 22.06.2019 05:00, Angelanova69134
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1
Do you know the correct answer?
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced eq...
Questions in other subjects:





Mathematics, 28.06.2019 18:30

Mathematics, 28.06.2019 18:30



Physics, 28.06.2019 18:30

English, 28.06.2019 18:30


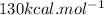
 is mentioned because it is for per mole of reaction. So for 4. moles of the product
is mentioned because it is for per mole of reaction. So for 4. moles of the product  we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.
we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.