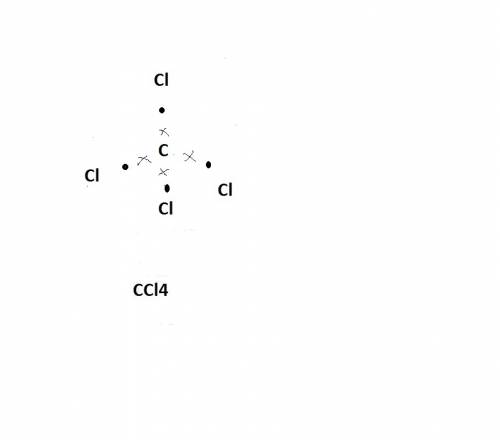
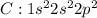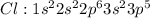Carbon tetrachloride has been widely used in the cleaning industry, in fire extinguishers, and as a refrigerant. construct an explanation of how carbon and chlorine combine to form carbon tetrachloride. a) nonmetal carbon shares valence electrons with each nonmetal chlorine forming four covalent bonds. b) nonmetal carbon loses a valence electron and chlorine metal gains a valence electron to form an ionic bond. c) carbon and chlorine are nonmetals and they shares their valence electrons to become ions and form ionic bonds. d) chlorine metal loses a valence electron to become a cation and nonmetal carbon gains a valence electron to become an anion forming a covalent bond.

Answers: 1
Similar questions

Chemistry, 27.06.2019 22:30, yehnerthannah
Answers: 1

Chemistry, 28.06.2019 03:30, 25linm
Answers: 1

Chemistry, 11.07.2019 16:00, krystalhurst97
Answers: 1

Chemistry, 15.07.2019 22:50, chelseal19847
Answers: 3
Do you know the correct answer?
Carbon tetrachloride has been widely used in the cleaning industry, in fire extinguishers, and as a...
Questions in other subjects:

English, 28.10.2019 19:31

English, 28.10.2019 19:31



English, 28.10.2019 19:31

Health, 28.10.2019 19:31




History, 28.10.2019 19:31