
Chemistry, 27.06.2019 19:30, ccarwile01
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. (4 points) a + b yieldsproducts trial [a] [b] rate 1 0.10 m 0.20 m 1.2 × 10-2 m/min 2 0.10 m 0.40 m 4.8 × 10-2 m/min 3 0.20 m 0.40 m 9.6 × 10-2 m/min

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2


Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2
Do you know the correct answer?
Determine the rate law, including the values of the orders and rate law constant, for the following...
Questions in other subjects:

Mathematics, 05.10.2020 14:01


Mathematics, 05.10.2020 14:01



English, 05.10.2020 14:01

Social Studies, 05.10.2020 14:01


Mathematics, 05.10.2020 14:01


![k[A]^1[B]^2](/tpl/images/0024/4376/f6c70.png) , order with respect to A is 1, order with respect to B is 2 and total order is 3. Rate law constant is
, order with respect to A is 1, order with respect to B is 2 and total order is 3. Rate law constant is 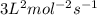
![Rate=k[A]^x[B]^y](/tpl/images/0024/4376/ddde1.png)
![1.2\times 10^{-2}=k[0.10]^x[0.20]^y](/tpl/images/0024/4376/c938a.png) (1)
(1)![4.8\times 10^{-2}=k[0.10]^x[0.40]^y](/tpl/images/0024/4376/a7ba9.png) (2)
(2)![\frac{4.8\times 10^{-2}}{1.2\times 10^{-2}}=\frac{k[0.10]^x[0.40]^y}{k[0.10]^x[0.20]^y}](/tpl/images/0024/4376/b4c1f.png)
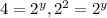 therefore y=2.
therefore y=2.![9.6\times 10^{-2}=k[0.20]^x[0.40]^y](/tpl/images/0024/4376/18d47.png) (4)
(4)![\frac{9.6\times 10^{-2}}{4.8\times 10^{-2}}=\frac{k[0.20]^x[0.40]^y}{k[0.10]^x[0.40]^y}](/tpl/images/0024/4376/61313.png)
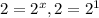 , x=1
, x=1![Rate=k[A]^1[B]^2](/tpl/images/0024/4376/ca297.png)
![1.2\times 10^{-2}=k[0.10]^1[0.20]^2](/tpl/images/0024/4376/f6a0d.png)
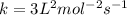 .
.



