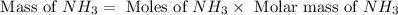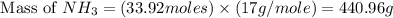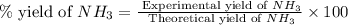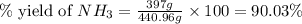Chemistry, 27.06.2019 20:30, elijahlylejamez45
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that will produce ammonia (nh3). hydrogen and nitrogen gases are reacted to produce the ammonia. for the first batch of ammonia production, 475 g of nitrogen is reacted with excess hydrogen, and 397 g of ammonia are produced. • write the balanced equation for the formation of ammonia from hydrogen and nitrogen.  2nh3 • calculate the theoretical yield of ammonia. work must be shown to earn credit. • calculate the percent yield for the ammonia production. work must be shown to earn credit.
2nh3 • calculate the theoretical yield of ammonia. work must be shown to earn credit. • calculate the percent yield for the ammonia production. work must be shown to earn credit.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, ReveenatheRaven2296
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 08:30, vanessadaniellet21
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1
Do you know the correct answer?
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that wil...
Questions in other subjects:




Physics, 29.08.2020 21:01


Mathematics, 29.08.2020 21:01

English, 29.08.2020 21:01




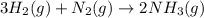
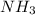 gas = 440.96 g
gas = 440.96 g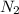 = 475 g
= 475 g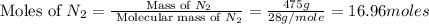
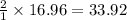 moles of
moles of