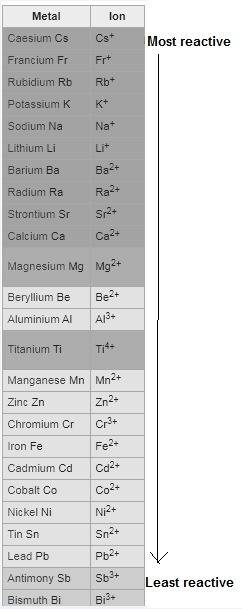
Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can replace aluminum (al) in the compound because lead is lower on the activity series. true false question 2(multiple choice worth 4 points) (04.03 mc) which of the following equations has the correct products and is balanced correctly for a reaction between na3po4 and koh? na3po4 + 3koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + kpo4, because k increases in charge from 1+ to 3+ when it is replaced na3po4 + koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + k3po4, because k increases in charge from 1+ to 3+ when it is replaced question 3(multiple choice worth 4 points) (04.03 lc) which of the following is a single replacement reaction? ba(oh)2 + h2so4 → baso4 + 2h2o 2mg + o2 → 2mgo h2o+ co2 → h2co3 zn + h2so4 → znso4 + h2 question 4(multiple choice worth 4 points) (04.03 mc) sodium metal reacts with water to produce hydrogen gas. what best describes this reaction? a single replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is more reactive than hydrogen. a single replacement reaction takes place because sodium is more reactive than hydrogen. question 5 (true/false worth 2 points) (04.03 lc) a single replacement reaction is a reaction in which one element replaces a similar element within a compound. true false question 6(multiple choice worth 4 points) (04.03 mc) the table shows the nature of reactants and products formed in a certain type of chemical reaction. nature of reactants and products reactants products metal + ionic compound metal + ionic compound which of the following is true about the type of chemical reaction? it is a single replacement reaction, and the anions in the two ionic compounds are different. it is a single replacement reaction, and the cations in the two ionic compounds are different. it is a double replacement reaction, and the anions in the two ionic compounds are different. it is a double replacement reaction, and the cations in the two ionic compounds are different.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, dijonmckenzie3
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1
Do you know the correct answer?
Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can re...
Questions in other subjects:



Mathematics, 26.02.2021 21:10

Geography, 26.02.2021 21:10

Mathematics, 26.02.2021 21:10

Mathematics, 26.02.2021 21:10

Biology, 26.02.2021 21:10

History, 26.02.2021 21:10


Biology, 26.02.2021 21:10


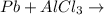 no reaction
no reaction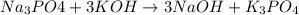 , because K retains the same charge throughout the reaction.
, because K retains the same charge throughout the reaction. 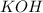 as well as
as well as 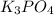
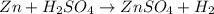
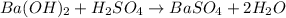 : is a double displacement reaction in which ion exchange takes place.
: is a double displacement reaction in which ion exchange takes place. 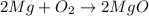 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.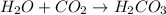 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.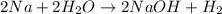 Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen.
Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen. 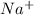 in NaOH and
in NaOH and 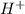 get reduced to give
get reduced to give 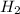
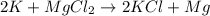 where K is a metal and
where K is a metal and 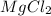 is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.
is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.